Potassium iodide
| Potassium iodide | |
|---|---|
 |
|
 |
|
|
Potassium iodide
|
|
| Identifiers | |
| CAS number | 7681-11-0 |
| PubChem | 4875 |
| RTECS number | TT2975000 |
| Properties | |
| Molecular formula | KI |
| Molar mass | 166.0028 g/mol |
| Appearance | white crystalline solid |
| Density | 3.123 g/cm3 |
| Melting point |
681 °C, 954 K, 1258 °F |
| Boiling point |
1330 °C, 1603 K, 2426 °F |
| Solubility in water | 128 g/100 ml (0 °C) 140 g/100 mL (20 °C) 176 g/100 mL (60°C) 206 g/100 mL (100°C) |
| Solubility | 2 g/100 mL (ethanol) soluble in acetone slightly soluble in ether, ammonia |
| Hazards | |
| MSDS | External MSDS |
| EU Index | Not listed |
| NFPA 704 |
 0
1
0
|
| Related compounds | |
| Other anions | Potassium fluoride Potassium chloride Potassium bromide |
| Other cations | Lithium iodide Sodium iodide Rubidium iodide Caesium iodide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Potassium iodide is an inorganic compound with formula KI. This white salt is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It is less hygroscopic than sodium iodide, making it easier to work with. Aged and impure samples are yellow because of oxidation of the iodide to iodine.[1]
- 4 KI + 2 CO2 + O2 → 2 K2CO3 + 2 I2
Potassium iodide is used medicinally in tablets, usually containing 130 mg of KI, of which 100 mg is iodine (as iodide). Potassium iodide may also be administered as a "saturated solution of potassium iodide" (SSKI) which in the U.S.P. generic formulation contains 1000 mg of KI per mL. This represents 333 mg KI and about 250 mg iodide, I - in a typical adult dose of 5 drops, assumed to be ⅓ mL.[2] Because SSKI is a viscous liquid, it is normally assumed to contain 15 drops/milliliter, not 20 drops/milliliter as is often assumed for water.[3] Thus, each drop of SSKI is assumed to contain about 50 mg iodine as iodide, I -. Two (2) drops of SSKI solution is equivalent to one 130 mg KI tablet (100 mg iodide).
Contents |
Structure, production, properties
Potassium iodide is ionic, K+I−. It crystallises in the sodium chloride structure. It is produced industrially by treating KOH with iodine.[1]
Inorganic chemistry
Since the iodide ion is a mild reducing agent, I − is easily oxidised to I2 by powerful oxidising agents such as chlorine:
- 2 KI(aq) + Cl2(aq) → 2 KCl + I2(aq)
This reaction is employed in the isolation of iodine from natural sources. Air will oxidize iodide, as evidenced by the observation of a purple extract when aged samples of KI are rinsed with dichloromethane. As formed under acidic conditions, hydroiodic acid (HI) is a stronger reducing agent.[4][5][6]
Like other iodide salts, KI forms I3− when combined with elemental iodine.
Unlike I2, I3− salts can be highly water-soluble. Through this reaction iodine is used in redox titrations. Aqueous KI3, "Lugol's solution," are used as disinfectants and as etchants for gold surfaces.
Potassium iodide is the precursor to silver(I) iodide, which is used for high speed photographic film:
Organic chemistry
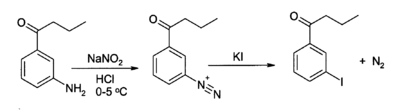
KI serves as a source of iodide in organic synthesis. A useful application is in the preparation of aryl iodides from arenediazonium salts.[7][8] For example:
KI, acting as a source of iodide, may also act as a nucleophilic catalyst for the alkylation of alkyl chlorides, bromides, or mesylates.
Applications
Industry
KI is a precursor to silver iodide (AgI) an important chemical in photography. KI is a component in some disinfectants and hair treatment hair chemicals. KI is also used as a fluorescence quenching agent in biomedical research, an application that takes advantage of collisional quenching of fluoresent substances by the iodide ion. Potassium iodide is a component in the electrolyte of dye sensitized solar cells (DSSC) along with iodine.
Nutrition
The major uses of KI include use as a nutritional supplement in animal feeds and also the human diet. For the latter, it is the most common additive used to "iodize" table salt (a public health measure to prevent iodine deficiency in populations which get little seafood). The oxidation of iodide causes slow loss of iodine content from iodised salts that are exposed to excess air. The alkali metal iodide salt, over time and exposure to excess oxygen and carbon dioxide, slowly oxidizes to metal carbonate and elemental iodine, which then evaporates.[9]
SSKI and pharmaceutical applications
Since potassium iodide is highly soluble in water, a saturated solution of potassium iodide, abbreviated SSKI, contains 1 gram (1000 mg) KI per milliliter (mL) of solution. This is less than 100% by weight, because SSKI is significantly more dense than pure water. Because KI is about 76.4% iodide by weight, SSKI contains about 764 mg iodide per mL, which is usually rounded to 750 mg/mL for convenience (50 mg iodide per drop, at 15 drops per mL typical of viscous liquids like SSKI).
- Saturated solutions of potassium iodide can be an emergency treatment for hyperthyroidism (so-called thyroid storm), as high amounts of iodide temporarily suppress secretion of thyroxine from the thyroid gland. The dose typically begins with a loading dose, then 1/3 mL (5 drops = 250 mg iodide) SSKI, three times per day.
- Iodide solutions made from a few drops of SSKI added to drinks have also been used as expectorants to increase the water content of respiratory secretions and encourage effective coughing.
- SSKI has been proposed as a topical treatment for sporotrichosis, but no trials have been conducted to determine the efficacy or side effects of such treatment.[10]
- Potassium iodide has been used for symptomatic treatment of erythema nodosum patients for persistant lesions whose cause remains unknown. It has been used in cases erythema nodosum associated with Crohn's disease.[11]
Thyroid protection against radioiodine in certain pharmaceuticals

Thyroid iodine uptake blockade with potassium iodide is used in nuclear medicine scintigraphy and therapy with some radioiodinated compounds that are not targeted to the thyroid, such as iobenguane (MIBG), which used to image or treat neural tissue tumors, or iodinated fibrinognen, which is used in fibrinogen scans to investigate clotting. These compounds contain iodine, but not in the iodide form. However, since they may be ultimately metabolized or break down to radioactive iodide, it is common to administer non-radioactive potassium iodide to ensure that iodide from these radiopharmaceuticals is not sequestered by the normal affinity of the thryoid for iodide.
FDA-approved dosing of potassium iodide for this purpose with iobenguane, is as follows: infants less than 1 month old, 16 mg; children 1 month to 3 years, 32 mg; children 3 years to 18 years, 65 mg; adults 130 mg[12]. However, some sources recommend alternative dosing regimens[13].
Not all sources are in agreement on the necessary duration of thyroid blockade, although agreement appears to have been reached about the necessity of blockade for both scintigraphic and therapeutic applications of iobenguane. Commercially available iobenguane is labeled with iodine-123, and product labeling recommends administration of potassium iodide 1 hour prior to administration of the radiopharmaceutical for all age groups [14], while the European Associated of Nuclear Medicine recommends (for iobenguane labeled with either isotope,) that potassium iodide administration begin one day prior to radiopharmaceutical administration, and continue until the day following the injection, with the exception of new-borns, who do not require potassium iodide doses following radiopharmaceutical injection[13].
Product labeling for diagnostic iodine-131 iobenguane recommends potassium iodide administration one day before injection and continuing 5 to 7 days following administration, in keeping with the much longer half-life of this isotope and its greater danger to the thyroid.[15]. Iodine-131 iobenguane used for therapeutic purposes requires a different pre-medication duration, beginning 24–48 hours prior to iobenguane injection and continuing 10–15 days following injection[16].
Thyroid protection from iodine-131 in fission accidents and emergencies
SSKI may be used in radioiodine-contamination emergencies (i.e., nuclear accidents) to "block" the thyroid's uptake of radioiodine (this is not the same as blocking the thyroid's release of thyroid hormone). The dose is smaller: 130 mg KI per day (100 mg iodide) which represents 2 drops of SSKI solution per day, for an adult.
Following the Chernobyl nuclear reactor disaster in April, 1986, a saturated solution of potassium iodide (SSKI) was administered to 10.5 million children and 7 million adults in Poland[17] as a prophylactic measure against accumulation of radioactive iodine-131 in the thyroid gland. People in the areas immediately surrounding Chernobyl itself, however, were not given the supplement.[18]
Potassium iodide was also approved in 1982 by the US FDA to protect the thyroid glands from radioactive iodine from accidents or fission emergencies. In the event of an accident or attack at a nuclear power plant, or fallout from a nuclear bomb, volatile fission product radionuclides may be released, of which 131I is one of the most common by-products and a particularly dangerous one due to thyroid gland concentration of it, which may lead to thyroid cancer. By saturating the body with a source of stable iodide prior to exposure, inhaled or ingested 131I tends to be excreted.
Potassium iodide cannot protect against any other causes of radiation poisoning, nor can it provide any degree of protection against dirty bombs that produce radionuclides other than isotopes of iodine.
| Age | KI in mg |
|---|---|
| Over 12 years old | 130 |
| 3 – 12 years old | 65 |
| 1 – 36 months old | 32 |
| < 1 month old | 16 |
See fission products and the external links for more details.
Potassium iodide’s (KI) value as a radiation protective (thyroid blocking) agent was demonstrated at the time of the Chernobyl nuclear accident when Soviet authorities distributed it in a 30 km zone around the plant. The purpose was to protect residents from radioactive iodine, a highly carcinogenic material found in nuclear reactors which had been released by the damaged reactor. Only a limited amount of KI was available, but those who received it were protected. Later, the US Nuclear Regulatory Commission (NRC) reported, “thousands of measurements of I-131 (radioactive iodine) activity…suggest that the observed levels were lower than would have been expected had this prophylactic measure not been taken. The use of KI…was credited with permissible iodine content in 97% of the evacuees tested.”[20]
Poland, 300 miles from Chernobyl, also distributed KI to protect its population. Approximately 18 million doses were distributed, with follow-up studies showing no known thyroid cancer among KI recipients.[21] With the passage of time, people living in irradiated areas where KI was not available have developed thyroid cancer at epidemic levels, which is why the US Food and Drug Administration (FDA) reported “The data clearly demonstrate the risks of thyroid radiation…KI can be used [to] provide safe and effective protection against thyroid cancer caused by irradiation.[22]
Chernobyl also demonstrated that the need to protect the thyroid from radiation was greater than expected. Within ten years of the accident, it became clear that thyroid damage caused by released radioactive iodine was virtually the only adverse health effect that could be measured. As reported by the NRC, studies after the accident showed that “As of 1996, except for thyroid cancer, there has been no confirmed increase in the rates of other cancers, including leukemia, among the…public, that have been attributed to releases from the accident.”[23]
But equally important to the question of KI is the fact that radiation releases are not “local” events. Researchers at the World Health Organization accurately located and counted the cancer victims from Chernobyl and were startled to find that “the increase in incidence [of thyroid cancer] has been documented up to 500 km from the accident site…significant doses from radioactive iodine can occur hundreds of kilometers from the site, beyond emergency planning zones."[24] Consequently, far more people than anticipated were affected by the radiation, which caused the United Nations to report in 2002 that “The number of people with thyroid cancer…has exceeded expectations. Over 11,000 cases have already been reported.”[25]
These findings were consistent with studies of the effects of previous radiation releases. In 1945, millions of Japanese were exposed to radiation from nuclear weapons, and the effects can still be measured. Today, nearly half (44.8%) the survivors of Nagasaki studied have identifiable thyroid disease, with the American Medical Association reporting “it is remarkable that a biological effect from a single brief environmental exposure nearly 60 years in the past is still present and can be detected.”[26]
These events, as well as the development of thyroid cancer among residents in the North Pacific from radioactive fallout following the United States' nuclear weapons testing in the 1950s (on islands nearly 200 miles downwind of the tests) were instrumental in the decision by the FDA in 1978 to issue a request for the availability of KI for thyroid protection in the event of a release from a commercial nuclear power plant or weapons-related nuclear incident. Noting that KI’s effectiveness was “virtually complete” and finding that iodine in the form of potassium iodide (KI) was substantially superior to other forms including iodate (KIO3) in terms of safety, effectiveness, lack of side effects, and speed of onset, the FDA invited manufacturers to submit applications to produce and market KI.[27] Today, three companies (Anbex, Inc., Fleming Co, and Recip of Sweden) have met the strict FDA requirements for manufacturing and testing of KI, and they offer products (IOSAT, ThyroShield, and Thyro-Safe, respectively) which are available for purchase.
Adverse reactions
There have been some reports of potassium iodide treatment causing swelling of the parotid gland (one of the three glands which secrete saliva), due to its stimulatory effects on saliva production.[28]
A saturated solution of KI (SSKI) is typically given orally in adult doses of about 250 mg iodide several times a day (5 drops of SSKI assumed to be ⅓ mL) for thryoid blockage and occasionally as an expectorant. At these doses, and sometimes at much lower doses, side effects may include: acne, loss of appetite, or upset stomach (especially during the first several days, as the body adjusts to the medication). More severe side effects which require notification of a physician are: fever, weakness, unusual tiredness, swelling in the neck or throat, mouth sores, skin rash, nausea, vomiting, stomach pains, irregular heartbeat, numbness or tingling of the hands or feet, or a metallic taste in the mouth.[29]
Precautions
Mild irritant, wear gloves. Chronic overexposure can have adverse effects on the thyroid. Potassium iodide is a possible teratogen
References
- ↑ 1.0 1.1 Phyllis A. Lyday "Iodine and Iodine Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim
- ↑ http://www.drugs.com/mmx/sski.html
- ↑ http://wiki.answers.com/Q/20_drops_per_ml_or_15_drops_per_ml Viscous liquids have about 15 drops per mL, not 20
- ↑ N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, Pergamon Press, Oxford, UK, 1984
- ↑ Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990
- ↑ The Merck Index, 7th edition, Merck & Co., Rahway, New Jersey, 1960
- ↑ L. G. Wade, Organic Chemistry, 5th ed., pp. 871-2, Prentice Hall, Upper Saddle RIver, New Jersey, 2003.
- ↑ J. March, Advanced Organic Chemistry, 4th ed., pp. 670-1, Wiley, New York, 1992.
- ↑ Katarzyna Waszkowiak & Krystyna Szymandera-Buszka. Effect of storage conditions on potassium iodide stability in iodised table salt and collagen preparations. International Journal of Food Science & Technology. Volume 43 Issue 5, Pages 895 -899. (Published Online: 27 Nov 2007)
- ↑ Xue, S.; Gu, R.; Wu, T.; Zhang, M.; Wang, X.; Wu, Taixiang (2009). "Oral potassium iodide for the treatment of sporotrichosis". Cochrane database of systematic reviews (Online) (4): CD006136. doi:10.1002/14651858.CD006136.pub2. PMID 19821356
- ↑ Marshall, JK; Irvine, EJ (September 1997). "Successful therapy of refractory erythema nodosum associated with Crohn's disease using potassium iodide.". Can J Gastroenterol 11 (6): 501–2. PMID 9347164.
- ↑ Kowalsky RJ, Falen, SW. Radiopharmaceuticals in Nuclear Pharmacy and Nuclear Medicine. 2nd ed. Washington DC: American Pharmacists Association; 2004.
- ↑ 13.0 13.1 https://www.eanm.org/scientific_info/guidelines/gl_paed_mibg.pdf?PHPSESSID=46d05b62d235c36a12166bf939b656c7
- ↑ http://nuclearpharmacy.uams.edu/resources/adreview.pdf
- ↑ Iobenguane Sulfate I 131 Injection Diagnostic package insert. Bedford, MA: CIS-US, Inc. July 1999.
- ↑ https://www.eanm.org/scientific_info/guidelines/gl_radio_ther_benzyl.pdf?PHPSESSID=46d05b62d235c36a12166bf939b656c7
- ↑ [1] US FDA, "Potassium Iodide as a Thyroid Blocking Agent in Radiation Emergencies," U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER); December, 2001.
- ↑ http://www.ecolo.org/documents/documents_in_english/Causes.ChernobyJF.doc
- ↑ Guidelines for Iodine Prophylaxis following Nuclear Accidents, World Health Organization, Update 1999
- ↑ US Nuclear Regulatory Commission, Report on the Accident at the Chernobyl Nuclear Power Station, NUREG-1250.
- ↑ "Iodine Prophylaxis in Poland After the Chernobyl Reactor Accident: Benefits and Risks". American Journal of Medicine 94. 1993.
- ↑ US Food and Drug Administration, FDA Talk Paper: Guidance on Protection Against Thyroid Cancer in Case of a Nuclear Accident
- ↑ US Nuclear Regulatory Commission, Assessment of the Use of Potassium Iodide (KI) As a Public Protective Action During Severe Reactor Accidents Quoting Thyroid Cancer in Children of Belarus Following the Chernobyl Accident, NUREG-1633
- ↑ World Health Organization, Guidelines for Iodine Prophylaxis Following Nuclear Accidents, Update 1999. World Health Organization, Geneva
- ↑ United Nations: Office for the Coordination of Humanitarian Affairs (OCHA), Chernobyl, a Continuing Catastrophe, New York and Geneva, 2000
- ↑ "Thyroid Disease 60 Years After Hiroshima and 20 Years After Chernobyl". JAMA 295 (9). 2006.
- ↑ US Federal Register, Vol. 43, No. 242, Dec 15, 1978.
- ↑ McCance; Huether. "Pathophysiology: The biological basis for disease in Adults and Children". 5th Edition. Elsievier Publishing
- ↑ http://www.medicinenet.com/potassium_iodide-oral/article.htm
External links
|
|||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||